The Process by Which There Is Programmed Cellular Death Is _____.
The cells of a multicellular organism are members of a highly organized community. The number of cells in this community is tightly regulated—not simply by controlling the rate of cell division, but also by controlling the rate of cell death. If cells are no longer needed, they commit suicide by activating an intracellular death program. This process is therefore called programmed cell death, although it is more commonly called apoptosis (from a Greek word meaning "falling off," as leaves from a tree).
The amount of apoptosis that occurs in developing and adult animal tissues can be astonishing. In the developing vertebrate nervous system, for example, up to half or more of the nerve cells normally die soon after they are formed. In a healthy adult human, billions of cells die in the bone marrow and intestine every hour. It seems remarkably wasteful for so many cells to die, especially as the vast majority are perfectly healthy at the time they kill themselves. What purposes does this massive cell death serve?
In some cases, the answers are clear. Mouse paws, for example, are sculpted by cell death during embryonic development: they start out as spadelike structures, and the individual digits separate only as the cells between them die (Figure 17-35). In other cases, cells die when the structure they form is no longer needed. When a tadpole changes into a frog, the cells in the tail die, and the tail, which is not needed in the frog, disappears (Figure 17-36). In many other cases, cell death helps regulate cell numbers. In the developing nervous system, for example, cell death adjusts the number of nerve cells to match the number of target cells that require innervation. In all these cases, the cells die by apoptosis.

Figure 17-35
Sculpting the digits in the developing mouse paw by apoptosis. (A) The paw in this mouse embryo has been stained with a dye that specifically labels cells that have undergone apoptosis. The apoptotic cells appear as bright green dots between the developing (more...)

Figure 17-36
Apoptosis during the metamorphosis of a tadpole into a frog. As a tadpole changes into a frog, the cells in the tadpole tail are induced to undergo apoptosis; as a consequence, the tail is lost. All the changes that occur during metamorphosis, including (more...)
In adult tissues, cell death exactly balances cell division. If this were not so, the tissue would grow or shrink. If part of the liver is removed in an adult rat, for example, liver cell proliferation increases to make up the loss. Conversely, if a rat is treated with the drug phenobarbital—which stimulates liver cell division (and thereby liver enlargement)—and then the phenobarbital treatment is stopped, apoptosis in the liver greatly increases until the liver has returned to its original size, usually within a week or so. Thus, the liver is kept at a constant size through the regulation of both the cell death rate and the cell birth rate.
In this short section, we describe the molecular mechanisms of apoptosis and its control. In the final section, we consider how the extracellular control of cell proliferation and cell death contributes to the regulation of cell numbers in multicellular organisms.
Apoptosis Is Mediated by an Intracellular Proteolytic Cascade
Cells that die as a result of acute injury typically swell and burst. They spill their contents all over their neighbors—a process called cell necrosis—causing a potentially damaging inflammatory response. By contrast, a cell that undergoes apoptosis dies neatly, without damaging its neighbors. The cell shrinks and condenses. The cytoskeleton collapses, the nuclear envelope disassembles, and the nuclear DNA breaks up into fragments. Most importantly, the cell surface is altered, displaying properties that cause the dying cell to be rapidly phagocytosed, either by a neighboring cell or by a macrophage (a specialized phagocytic cell, discussed in Chapter 24), before any leakage of its contents occurs (Figure 17-37). This not only avoids the damaging consequences of cell necrosis but also allows the organic components of the dead cell to be recycled by the cell that ingests it.
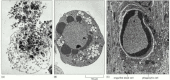
Figure 17-37
Cell death. These electron micrographs show cells that have died by (A) necrosis or (B and C) apoptosis. The cells in (A) and (B) died in a culture dish, whereas the cell in (C) died in a developing tissue and has been engulfed by a neighboring cell. (more...)
The intracellular machinery responsible for apoptosis seems to be similar in all animal cells. This machinery depends on a family of proteases that have a cysteine at their active site and cleave their target proteins at specific aspartic acids. They are therefore called caspases. Caspases are synthesized in the cell as inactive precursors, or procaspases, which are usually activated by cleavage at aspartic acids by other caspases (Figure 17-38A). Once activated, caspases cleave, and thereby activate, other procaspases, resulting in an amplifying proteolytic cascade (Figure 17-38B). Some of the activated caspases then cleave other key proteins in the cell. Some cleave the nuclear lamins, for example, causing the irreversible breakdown of the nuclear lamina; another cleaves a protein that normally holds a DNA-degrading enzyme (a DNAse) in an inactive form, freeing the DNAse to cut up the DNA in the cell nucleus. In this way, the cell dismantles itself quickly and neatly, and its corpse is rapidly taken up and digested by another cell.
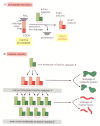
Figure 17-38
The caspase cascade involved in apoptosis. (A) Each suicide protease is made as an inactive proenzyme (procaspase), which is usually activated by proteolytic cleavage by another member of the caspase family. As indicated, two of the cleaved fragments (more...)
Activation of the intracellular cell death pathway, like entry into a new stage of the cell cycle, is usually triggered in a complete, all-or-none fashion. The protease cascade is not only destructive and self-amplifying but also irreversible, so that once a cell reaches a critical point along the path to destruction, it cannot turn back.
Procaspases Are Activated by Binding to Adaptor Proteins
All nucleated animal cells contain the seeds of their own destruction, in the form of various inactive procaspases that lie waiting for a signal to destroy the cell. It is therefore not surprising that caspase activity is tightly regulated inside the cell to ensure that the death program is held in check until needed.
How are procaspases activated to initiate the caspase cascade? A general principle is that the activation is triggered by adaptor proteins that bring multiple copies of specific procaspases, known as initiator procaspases, close together in a complex or aggregate. In some cases, the initiator procaspases have a small amount of protease activity, and forcing them together into a complex causes them to cleave each other, triggering their mutual activation. In other cases, the aggregation is thought to cause a conformational change that activates the procaspase. Within moments, the activated caspase at the top of the cascade cleaves downstream procaspases to amplify the death signal and spread it throughout the cell (see Figure 17-38B).
Procaspase activation can be triggered from outside the cell by the activation of death receptors on the cell surface. Killer lymphocytes (discussed in Chapter 24), for example, can induce apoptosis by producing a protein called Fas ligand, which binds to the death receptor protein Fas on the surface of the target cell. The clustered Fas proteins then recruit intracellular adaptor proteins that bind and aggregate procaspase-8 molecules, which cleave and activate one another. The activated caspase-8 molecules then activate downstream procaspases to induce apoptosis (Figure 17-39A). Some stressed or damaged cells kill themselves by producing both the Fas ligand and the Fas protein, thereby triggering an intracellular caspase cascade.
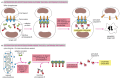
Figure 17-39
Induction of apoptosis by either extracellular or intracellular stimuli. (A) Extracellular activation. A killer lymphocyte carrying the Fas ligand binds and activates Fas proteins on the surface of the target cell. Adaptor proteins bind to the intracellular (more...)
When cells are damaged or stressed, they can also kill themselves by triggering procaspase aggregation and activation from within the cell. In the best understood pathway, mitochondria are induced to release the electron carrier protein cytochrome c (see Figure 14-26) into the cytosol, where it binds and activates an adaptor protein called Apaf-1 (Figure 17-39B). This mitochondrial pathway of procaspase activation is recruited in most forms of apoptosis to initiate or to accelerate and amplify the caspase cascade. DNA damage, for example, as discussed earlier, can trigger apoptosis. This response usually requires p53, which can activate the transcription of genes that encode proteins that promote the release of cytochrome c from mitochondria. These proteins belong to the Bcl-2 family.
Bcl-2 Family Proteins and IAP Proteins Are the Main Intracellular Regulators of the Cell Death Program
The Bcl-2 family of intracellular proteins helps regulate the activation of procaspases. Some members of this family, like Bcl-2 itself or Bcl-X L , inhibit apoptosis, at least partly by blocking the release of cytochrome c from mitochondria. Other members of the Bcl-2 family are not death inhibitors, but instead promote procaspase activation and cell death. Some of these apoptosis promoters, such as Bad, function by binding to and inactivating the death-inhibiting members of the family, whereas others, like Bax and Bak, stimulate the release of cytochrome c from mitochondria. If the genes encoding Bax and Bak are both inactivated, cells are remarkably resistant to most apoptosis-inducing stimuli, indicating the crucial importance of these proteins in apoptosis induction. Bax and Bak are themselves activated by other apoptosis-promoting members of the Bcl-2 family such as Bid.
Another important family of intracellular apoptosis regulators is the IAP (inhibitor of apoptosis) family. These proteins are thought to inhibit apoptosis in two ways: they bind to some procaspases to prevent their activation, and they bind to caspases to inhibit their activity. IAP proteins were originally discovered as proteins produced by certain insect viruses, which use them to prevent the infected cell from killing itself before the virus has had time to replicate. When mitochondria release cytochrome c to activate Apaf-1, they also release a protein that blocks IAPs, thereby greatly increasing the efficiency of the death activation process.
The intracellular cell death program is also regulated by extracellular signals, which can either activate apoptosis or inhibit it. These signal molecules mainly act by regulating the levels or activity of members of the Bcl-2 and IAP families. We see in the next section how these signal molecules help multicellular organisms regulate their cell numbers.
Summary
In multicellular organisms, cells that are no longer needed or are a threat to the organism are destroyed by a tightly regulated cell suicide process known as programmed cell death, or apoptosis. Apoptosis is mediated by proteolytic enzymes called caspases, which trigger cell death by cleaving specific proteins in the cytoplasm and nucleus. Caspases exist in all cells as inactive precursors, or procaspases, which are usually activated by cleavage by other caspases, producing a proteolytic caspase cascade. The activation process is initiated by either extracellular or intracellular death signals, which cause intracellular adaptor molecules to aggregate and activate procaspases. Caspase activation is regulated by members of the Bcl-2 and IAP protein families.

The Process by Which There Is Programmed Cellular Death Is _____.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26873/#:~:text=In%20multicellular%20organisms%2C%20cells%20that,programmed%20cell%20death%2C%20or%20apoptosis.
0 Response to "The Process by Which There Is Programmed Cellular Death Is _____."
Publicar un comentario